Why do real molecules have different bond angles than models?
Table of Contents
- Why do real molecules have different bond angles than models?
- What determines the bond angle in a molecule?
- Why do different molecules get different molecular shapes?
- Why is 109.5 The ideal bond angle?
- What is the ideal bond angle?
- How do you compare bond angles?
- How do you find the largest bond angle?
- How do you know if a molecule is tetrahedral?
- What is the smallest molecule?
- Which has highest bond angle?
- What do bond angles tell us about molecules?
- How does the presence of a lone pair affect the bond angle?
- What's the difference between a model and a molecule?
- What causes the bond angle of an atom to decrease?
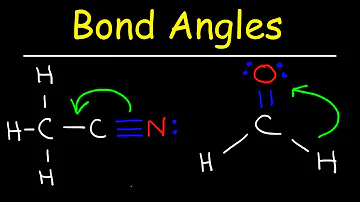
Why do real molecules have different bond angles than models?
Because a lone pair of electrons requires more space than a bonding pair, the molecular geometry distorts to afford the lone pair greater space, with the result that bond angles are smaller than those of the ideal geometry.
What determines the bond angle in a molecule?
The bond angles depend on the number of lone electron pairs. For example, boron trichloride has no lone pairs, a trigonal planar shape and bond angles of 120 degrees. The trioxygen molecule O3 has one lone pair and forms a bent shape with bond angles of 118 degrees.
Why do different molecules get different molecular shapes?
Molecules assume different shapes due to patterns of shared and unshared electrons. In these examples all electrons affecting the shape of the molecules are shared in the covalent bonds holding the atoms together to form the molecules.
Why is 109.5 The ideal bond angle?
VSEPR Theory: Valence Shell Electron Pair Repulsion Theory. ... VSEPR theory predicts methane is a perfect tetrahedron with all H-C-H bond angles equal at 109.5o, because the hydrogen atoms repel equally, and because this geometry puts the greatest distance between all four bonded electrons pairs.
What is the ideal bond angle?
The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the VSEPR theory. Essentially, bond angles is telling us that electrons don't like to be near each other.
How do you compare bond angles?
As electronegativity increases from left to right across the period so F atom is more electronegative than O atom. The greater the repulsion of bonding atoms greater is the bond angle.
How do you find the largest bond angle?
1:205:27Predicting Bond Angles - YouTubeYouTube
How do you know if a molecule is tetrahedral?
For example, a molecule with two electron pairs (and no lone pairs) around the central atom has a linear shape, and one with four electron pairs (and no lone pairs) around the central atom would have a tetrahedral shape.
What is the smallest molecule?
The smallest molecule is diatomic hydrogen (H2), with a bond length of 0.74 angstroms. Macromolecules are large molecules composed of smaller subunits; this term from biochemistry refers to nucleic acids, proteins, carbohydrates, and lipids.
Which has highest bond angle?
Hence, ammonia has the highest bond angle.
What do bond angles tell us about molecules?
The bond angle can help differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the VSEPR theory. Essentially, bond angles is telling us that electrons don't like to be near each other.
How does the presence of a lone pair affect the bond angle?
1 Answer. Lone pair repulsion: Bond angle is affected by the presence of lone pair of electrons at the central atom. A lone pair of electrons at the central atom always tries to repel the shared pair (bonded pair) of electrons. Due to this, the bonds are displaced slightly inside resulting in a decrease of bond angle.
What's the difference between a model and a molecule?
Then, compare the model to real molecules! Recognize that molecule geometry is due to repulsions between electron groups. Recognize the difference between electron and molecular geometry. Name molecule and electron geometries for molecules with up to six electron groups surrounding a central atom.
What causes the bond angle of an atom to decrease?
1 Answer. A lone pair of electrons at the central atom always tries to repel the shared pair (bonded pair) of electrons. Due to this, the bonds are displaced slightly inside resulting in a decrease of bond angle. Electronegativity: If the electronegativity of the central atom decreases, bond angle decreases.

 Main Topics
Main Topics