What is p pi p pi bond?
Table of Contents
- What is p pi p pi bond?
- Why does P pi not form p pi bonds?
- What is P Bond?
- Which of the following molecule is not having p pi D pi bonding?
- Which element among the following does P Pi P Pi multiple bonds?
- Why heavier elements of group 15 do not form p pi p pi bonds?
- Is a pi bond a single bond?
- What is the meaning of P pi pi pi bond?
- What counts as a pi electron?
- How many P pi DPI bonds are there in Sulphur?
- How are three sigma bonds and one pi bond formed?

What is p pi p pi bond?
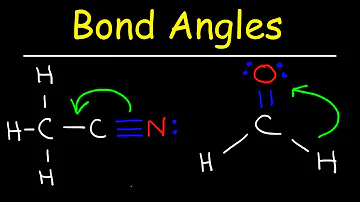
Pi bond: It is formed by lateral overlap of the orbitals. P - Pi p - Pi bond is formed when one p orbital of each combining atom is overlapped laterally. P - Pi d - Pi bond is formed when p orbital of one atom and d orbital of another atom overlap laterally.
Why does P pi not form p pi bonds?
Nitrogen, being smaller in size, can effectively form p pi – p pi bonds with other atoms of itself and atoms of other elements with a small size and high electro-negativity such as oxygen and carbon. The other elements do not form p pi - p pi bonds because of their relatively larger size.
What is P Bond?
In chemistry, pi bonds (π bonds) are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally. Each of these atomic orbitals has zero electron density at a shared nodal plane, passing through the two bonded nuclei.
Which of the following molecule is not having p pi D pi bonding?
There is a pπ-pπ donation of filled nitrogen pz atomic orbital to adjacent Boron pz atomic orbitals. So, borazine contain 3 pi and 12 sigma bonds. Hence, there is no involvement of any d-orbital in hybridization of B3N3H6.
Which element among the following does P Pi P Pi multiple bonds?
Among all the group-14 elements carbon exhibits pπ-pπ multiple bonding.
Why heavier elements of group 15 do not form p pi p pi bonds?
Carbon has the unique ability to form pπ – pπ multiple bond with itself and with other atoms of small size and high electro negativity whereas heavier elements do not from pπ – pπ bonds because their atomic orbital's are too large and diffuse to have effective overlapping.
Is a pi bond a single bond?
In general, single bonds between atoms are always sigma bonds. Double bonds are comprised of one sigma and one pi bond. Triple bonds are comprised of one sigma bond and two pi bonds.
What is the meaning of P pi pi pi bond?
Eg. in BF3, B has incomplete octet so a fully filled p (pi) orbital of F donates a lone pair of e to the vacant orbital of B. so here pp (pi)-p (pi) bonding occurs and is known as back-bonding also. Sigma bond : It is formed by the end to end overlap of orbitals of two atoms which are combining.
What counts as a pi electron?
1 Answer. Truong-Son N. #pi# bonds are simply the second bond made in a double bond. Any pure double bond is one sigma/#sigma# and one pi/#pi# bond. Since any one chemical bond (meaning only one line in bond line notation) contains at most two electrons, you can count two #pi# electrons per double bond, and ignore the #sigma# electrons.
How many P pi DPI bonds are there in Sulphur?
> The number of p pi - dpi b... is sp2 hybridized. Three σ bonds are formed between the three sp2 hybridised orbital of sulphur and 3 pure p orbitals of three oxygen atoms. We have one pure p orbital and two pure d orbitals in sulphur.
How are three sigma bonds and one pi bond formed?
The sp 2 hybridized carbons now form three sigma bonds and one pi bond, as illustrated below. The sp 2 hybridized orbital in the carbon atom is made up of a 2s electron, a 2p x electron, and a 2p y electron. It can form a total of three sigma bonds. The 2p z electrons of the carbon atoms now form a pi bond with each other.

 Main Topics
Main Topics