What causes bond angles to increase?
Table of Contents
- What causes bond angles to increase?
- What happens to the bond angles as the number of bonds increase?
- What happens to the bond angle as more atoms are bonded to the central atom?
- Why do bond angles decrease down the group?
- What factor causes bond angles to decrease?
- Do any of the geometry names change if you use double or triple bonds instead of single bonds?
- Why do bond angles decrease in hydrides of group 16?
- Why does George want to be in charge of Lennie?
- Why is Lennie so dangerous to George Steinbeck?
- How did Lennie break her neck in of mice and men?
- How is the ideal angle for a hydrogen bond determined?
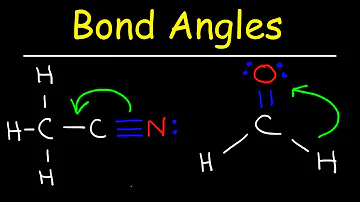
What causes bond angles to increase?
As more electron density remains on the central atom, electron repulsion between the bonded pairs increases and bond angles increase.
What happens to the bond angles as the number of bonds increase?
iii) The bond angle decreases with increase in the size of central atom. ... However the bond angle increases with increase in the size of ligand atoms, which surround the central atom. There is less repulsion between smaller ligand atoms and they can move closer to each other and thus decrease the bond angle.
What happens to the bond angle as more atoms are bonded to the central atom?
As the size of the central atom increases, the bond angles decrease; thus, we observe a negative relationship between size of the central atom and the bond angle in these molecules.
Why do bond angles decrease down the group?
Dipole moment is proportional to electronegativity of the central atom. So it decreases down the group. These hydrides are pyramidal in shape with a lone pair of electrons in one of the orbits. The bond angle gradually decreases down the group due to the decrease in bond pair- bond pair repulsions.
What factor causes bond angles to decrease?
Lone pair repulsion: Bond angle is affected by the presence of lone pair of electrons at the central atom. A lone pair of electrons at the central atom always tries to repel the shared pair (bonded pair) of electrons. Due to this, the bonds are displaced slightly inside resulting in a decrease of bond angle.
Do any of the geometry names change if you use double or triple bonds instead of single bonds?
No, using double or triple bonds did not change the geometry names of the molecules. 8. The phrase 'electron domain' (text uses the terms 'electron group') is used in discussions of molecular geometry to mean either a lone pair or a bond on the central atom of a molecule.
Why do bond angles decrease in hydrides of group 16?
The bond angle in the hydrides of the group 16 decreases as we go down the group. As electronegativity falls down the group, the tendency of the central atom to attract bond pair of electrons falls thus making lone pair - lone pair repulsion more important and thereby reducing the bond angle.
Why does George want to be in charge of Lennie?
Here the reader sees that George enjoys the opportunity to not only give Lennie advice, but also to be in charge. Lennie gives George stature. But now George uses that power carefully; he respects the fact that Lennie is not mean and would never intentionally hurt anyone.
Why is Lennie so dangerous to George Steinbeck?
Lennie gives George stature. But now George uses that power carefully; he respects the fact that Lennie is not mean and would never intentionally hurt anyone. What George does not seem to realize is how dangerous Lennie's strength can be, a danger that Steinbeck makes clear when Lennie crushes Curley's hand.
How did Lennie break her neck in of mice and men?
Lennie becomes frightened, and unintentionally breaks her neck thereafter and runs away. When the other ranch hands find the corpse, George realizes that their dream is at an end. George hurries to find Lennie, hoping he will be at the meeting place they designated in case he got into trouble.
How is the ideal angle for a hydrogen bond determined?
The ideal bond angle depends on the nature of the hydrogen bond donor. The following hydrogen bond angles between a hydrofluoric acid donor and various acceptors have been determined experimentally: Strong hydrogen bonds are revealed by downfield shifts in the 1 H NMR spectrum.

 Main Topics
Main Topics