Why are reactions of higher order less in number?
Table of Contents
- Why are reactions of higher order less in number?
- What is 3rd order reaction?
- What is the order of reaction if quite rare?
- Can a reaction have zero activation energy?
- Which rate law is 3rd order overall?
- How will the reaction rate change if A is doubled?
- What is highest order of reaction?
- When does a reaction have more than one order?
- When does a reaction have a zero order?
- Which is an example of a third order reaction?
- Why is the Order of chemical reactions important?
Why are reactions of higher order less in number?
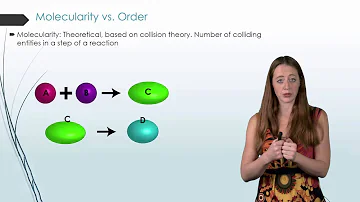
A reaction takes place because molecules collide. The chances for a large number of molecules or ions to collide ismultaneously are less. Hence, the reactions of higher order are less.
What is 3rd order reaction?
: a chemical reaction in which the rate of reaction is proportional to the concentration of each of three reacting molecules — compare order of a reaction.
What is the order of reaction if quite rare?
Due to this very low probability of colliding of molecules, the higher order reactions (> 3) are quite rare.
Can a reaction have zero activation energy?
This implies that every collision results into a chemical reaction which cannot be true. So, a reaction cannot have zero activation energy.
Which rate law is 3rd order overall?
Reaction Order and Rate Constant Units
| Overall Reaction Order (x) | Rate Constant Unit (Lx−1 mol1−x s−1) |
|---|---|
| 0 (zero) | mol L−1 s−1 |
| 1 (first) | s−1 |
| 2 (second) | L mol−1 s−1 |
| 3 (third) | L2 mol−2 s−1 |
How will the reaction rate change if A is doubled?
Second order When you double the concentration the rate doubles. The rate is proportional to the square of the concentration of a reactant. When you double the concentration the rate goes up four times. The rate is not affected by the concentration of a reactant.
What is highest order of reaction?
Mostly the order of the maximum chemical reactions is either o or one or two only. Order of the reaction also depends on the number of reactants they are going to collide each other during the reaction.
When does a reaction have more than one order?
A reaction can have more than one order depending upon different concentration of reactants. For example, a reaction having rate law is found to have a zero order initially when reactants are in high concentration, while the reaction order shifts to first order at the end of reaction when concentration of reactant is low.
When does a reaction have a zero order?
is found to have a zero order initially when reactants are in high concentration, while the reaction order shifts to first order at the end of reaction when concentration of reactant is low. A zero order reaction is the one whose rate is independent of concentration of reactant. Rate law for such reaction is − r a = k C a 0 = k. = k.
Which is an example of a third order reaction?
Reaction orders are determined experimentally, and can be fractional negative, zero or positive numbers. For example, the Wikipedia page for "reaction order" gives the chemical reaction between mercury (II) chloride and the oxalate ion as an example of a third-order reaction, whereby the rate depends on the concentration of 3 reactant terms.
Why is the Order of chemical reactions important?
Second order reaction involves determination of concentration of both reactants simultaneously. This method of pseudo first order reaction is useful in the study of chemical kinetics. If one of the reactants is expensive, then the other reactant can be taken in excess quantity and the reaction order with respect...

 Main Topics
Main Topics