Why does lithium have a high 2nd ionization energy?
Table of Contents
- Why does lithium have a high 2nd ionization energy?
- Which has a higher second ionization energy Li or be?
- Which element has the largest second ionization energy mg Na fo?
- Why does lithium have more ionization energy than sodium?
- Why is the second ionization energy of beryllium greater than lithium?
- Why is the second ionization energy higher than the first?
- How does the electron feel in lithium compared to hydrogen?
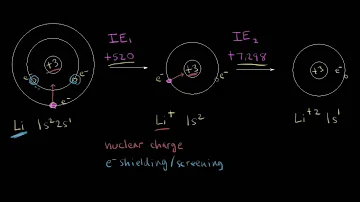
Why does lithium have a high 2nd ionization energy?
Second Ionisation Energies are always higher than the first due to two main reasons: You are removing the electron from a position that it slightly closer to the nucleus, and therefore is subject to greater attraction to the nucleus.
Which has a higher second ionization energy Li or be?
The second ionization energy is always larger than the first ionization energy, because it requires even more energy to remove an electron from a cation than it is from a neutral atom....Periodic Trends — Ionization Energy.
| 2 | |
| 1A | Li 513 |
| 2A | Be 899 |
| 4A | C 1086 |
Which element has the largest second ionization energy mg Na fo?
Hence, second ionization energy of sodium is largest.
Why does lithium have more ionization energy than sodium?
This is because of the increase in the nuclear charge due to addition of electrons . So due to the increase in the nuclear charge the nucleus pull the electrons which makes the electron difficult to move out of the shell..therefore we need more energy to remove an electron.
Why is the second ionization energy of beryllium greater than lithium?
But in case of the second electron, lithium has already gained stability by loosing one electron but beryllium can get octet configuration by loosing the second electron hence it shows more tendency than lithium to loose the second electron so its second ionization energy is lesser than that of lithium.
Why is the second ionization energy higher than the first?
Second Ionisation Energies are always higher than the first due to two main reasons: You are removing the electron from a position that it slightly closer to the nucleus, and therefore is subject to greater attraction to the nucleus.
How does the electron feel in lithium compared to hydrogen?
One can think of the electron as feeling a net 1+ pull from the center (3 protons offset by the two 1s 2 electrons). If you compare lithium with hydrogen (instead of with helium), the hydrogen's electron also feels a 1+ pull from the nucleus, but the distance is much greater with lithium.

 Main Topics
Main Topics


