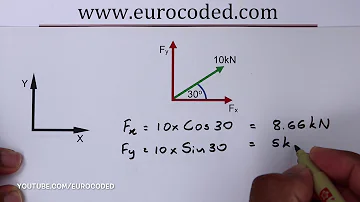
What is the equation and units of molarity?
Table of Contents
- What is the equation and units of molarity?
- What is molarity and its SI unit?
- What are the units of molarity molality and normality?
- What is the SI unit of force?
- What is the SI unit of volume?
- What is the SI unit of molarity Class 11?
- What is the SI unit of concentration?
- What is the unit of formality?
- Which is the unit of molarity of a solution?
- Which is the SI unit for molar concentration?
- What do you mean by molarity, molality and normality?
- How is the molar concentration of solute calculated?

What is the equation and units of molarity?
In chemistry, concentration of a solution is often measured in molarity (M), which is the number of moles of solute per liter of solution. This molar concentration (ci) is calculated by dividing the moles of solute (ni ) by the total volume (V) of the: ci=niV. The SI unit for molar concentration is mol/m3.
What is molarity and its SI unit?
Molarity (M) is defined as the number of moles of solute per liter of solution. Molarity is a measurement of the moles in the total volume of the solution. molarity = moles of solute/liters of solution. The S.I. unit is Mole/volume.
What are the units of molarity molality and normality?
Molarity, molality, and normality are all units of concentration in chemistry. Molarity ( ) is defined as the number of moles of solute per liter of solution. Molality ( ) is defined as the number of moles of solute per kilogram of solvent. Normality ( ) is defined as the number of equivalents per liter of solution.
What is the SI unit of force?
newton The SI unit of force is the newton, symbol N. The base units relevant to force are: The metre, unit of length — symbol m. The kilogram, unit of mass — symbol kg.
What is the SI unit of volume?
cubic meter The SI unit of volume is the cubic meter (m3), which is a derived unit.
What is the SI unit of molarity Class 11?
Therefore, the units of molarity is mol/L or mol. L−1 . Note: Molarity(M) and molality(m) should not be confused with Normality(N). Normality is also used to measure concentration of chemical solution but it is defined as the number of gram equivalents of solute present in one litre of a solution.
What is the SI unit of concentration?
The SI unit of concentration (of amount of substance) is the mole per cubic meter (mol/m3).
What is the unit of formality?
Formality is the number of formula mass in gram present per litre of a solution. If the formula mass of solute is equal to its molar mass, then the formality is equal to molarity. ... A mole of an ionic compound is called formole and its molarity is called formality.
Which is the unit of molarity of a solution?
Molarity is also known as the molar concentration of a solution. The units of molarity are M or mol/L. A 1 M solution is said to be “one molar.”
Which is the SI unit for molar concentration?
The SI unit for molar concentration is mol/m3. However, mol/L is a more common unit for molarity. A solution that contains 1 mole of solute per 1 liter of solution (1 mol/L) is called “one Molar” or 1 M. The unit mol/L can be converted to mol/m3 using the following equation:
What do you mean by molarity, molality and normality?
What do you mean by molarity, molality and normality? Molarity: Molarity, which is denoted by M, is defined as the number of moles of solute present in one litre of solution. It is given by the equation: molarity = no. of moles of solute/volume of solution in litres. Molality: Molality, denoted by m, is defined as the number of moles ...
How is the molar concentration of solute calculated?
Molar concentration or molarity is most commonly expressed in units of moles of solute per litre of solution. For use in broader applications, it is defined as amount of substance of solute per unit volume of solution, or per unit volume available to the species, represented by lowercase c {\displaystyle c} : [3]

 Main Topics
Main Topics