What is structure of ClF5?
Table of Contents
- What is structure of ClF5?
- How many bonds are in ClF5?
- What is the bond angle in ClF5?
- What is the formal charge of chlorine in ClF5?
- What is the hybridization of ClF5?
- How to calculate the hybridization of chlorine and PF5?
- What is the hybridization of the central atom in ClF5?
- What happens when CL combines with fluorine to form ClF3?
- What kind of bonds are formed during PCl5 hybridization?
What is structure of ClF5?
Infobox references. Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum.
How many bonds are in ClF5?
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The CHLORINE PENTAFLUORIDE molecule contains a total of 5 bond(s) There are 5 non-H bond(s).
What is the bond angle in ClF5?
There is no bond angle. In these cases, the electron-domain geometry is always trigonal bipyramidal. However, only the first molecule is that shape with the ideal angles of 90 and 120 degrees for the axial and equatorial bonds, respectively.
What is the formal charge of chlorine in ClF5?
So our formal charges are zero for each atom in ClF5.
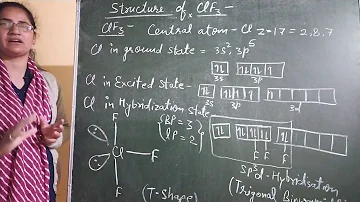
What is the hybridization of ClF5?
Accordingly, the hybridization of Cl in ClF5 is sp3d2.
How to calculate the hybridization of chlorine and PF5?
In case of ClF5 , chlorine is a central metal surrounded by five flourine atoms and lone pair we have to find , if any. 1s2, 2s2 , 2p6 ,3s2 , 3p5 means 7 electrons in valence shell . Therefore, total no. Of electron pairs in the valence shell of central Cl atom = (no.
What is the hybridization of the central atom in ClF5?
Halogens can mutually combine to form a number of covalent compounds that are called interhalogen compounds. In case of ClF5 , chlorine is a central metal surrounded by five flourine atoms and lone pair we have to find , if any.
What happens when CL combines with fluorine to form ClF3?
Now, when Cl needs to combine with Fluorine atoms to form ClF3 it needs three unpaired electrons to bond with three F-atoms. Here, one of the paired electrons of Cl in the 3p subshell remains as a lone pair or unpaired.
What kind of bonds are formed during PCl5 hybridization?
Types of bonds formed during the PCl5 hybridization-. Equatorial bonds: 3 P–Cl bond which lies in one plane to make an angle with each other. The angle made between them is 120°. Axial bonds: 2 P–Cl bonds where one lies above the equatorial plane and the other below the plane to make an angle with the plane. The angle made with the plane 90°.

 Main Topics
Main Topics


