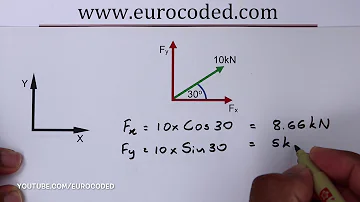
What is an empirical formula for glucose?
Table of Contents
- What is an empirical formula for glucose?
- What is the empirical formula of glucose Class 11?
- What are the empirical formulas for glucose and fructose?
- What is the empirical formula for C 6 H 12 O 3?
- What pair has the same empirical formula?
- What is the empirical formula of fructose?
- What is the empirical formula of c6h14?
- What is the empirical formula of C4H10?
- Which is the empirical formula for glucose CH2O?
- How to work out a molecular formula from an empirical formula?
- How to write an empirical formula in GCSE?
- How to write an empirical formula for 32?

What is an empirical formula for glucose?
Glucose is an important simple sugar that cells use as their primary source of energy. Its molecular formula is C6H12O6. Since each of the subscripts is divisible by 6, the empirical formula for glucose is CH2O.
What is the empirical formula of glucose Class 11?
The empirical formula of glucose would be the simplest whole number ratio of all the atoms in the compound. Thus, the empirical formula of glucose is \[C{H_2}O\]. Hence, the correct answer is (D).
What are the empirical formulas for glucose and fructose?
What is the empirical formula of fructose C6H12O6? So it is divided by 6 and so empirical formula is CH2O. Empirical formula = C4H5N2O.
What is the empirical formula for C 6 H 12 O 3?
The empirical formula of C6 H12 , the molecular formula of cyclohexane, is CH2. We determine the empirical formula by simplifying the molecular equation to the smallest ratio of atoms using the greatest common denominator of all the number. As 12 is a multiple of 6, we can use 6 as the greatest common denominator.
What pair has the same empirical formula?
Many compounds may have the same empirical formula. For example, formaldehyde, each molecule of which consists of one carbon atom, two hydrogen atoms, and one oxygen atom, has the molecular formula CH2O, which is identical to the empirical formula of glucose.
What is the empirical formula of fructose?
C6H12O6 Fructose/Formula The empirical formula of the fructose (C6H12O6) found in honey is the simplest formula so divide the chemical formula by 6. Thus, CH2O.
What is the empirical formula of c6h14?
C6H14 Hexane/Formula
What is the empirical formula of C4H10?
C₄H₁₀ Butane/Formula Its empirical formula is CH. Your formula C4H10 (most likely n-butane) is a molecular formula. The empirical formula fo that substance would be C2H5 (a ratio of 2 to 5). The answer is 14.
Which is the empirical formula for glucose CH2O?
The empirical formula for glucose is CH2O. An empirical formula represents the lowest whole number ratio of elements in a compound. The molecular formula for glucose is C6H12O6. The subscripts represent a multiple of an empirical formula. To determine the empirical formula, divide the subscripts by the GCF of 6, which gives CH2O.
How to work out a molecular formula from an empirical formula?
From the empirical formula, you can work out the molecular formula if you know the relative formula mass (Mr) of the compound. Add up the atomic masses of the atoms in the empirical formula. For example, the empirical formula of a hydrocarbon is CH2 and its Mr is 42. the mass of the atoms in the empirical formula is 14
How to write an empirical formula in GCSE?
Empirical formulae Step Action Result 1 Write the element symbols S O 2 Write the masses 3.2 g 6.4 g – 3.2 g = 3.2 g 3 Write the Ar values 32 16 4 Divide masses by Ar 3.2 ÷ 32 = 0.1 3.2 ÷ 16 = 0.2 2 more rows ...
How to write an empirical formula for 32?
Empirical formulae Step Action Result 3 Write the Ar values 32 16 4 Divide masses by Ar 3.2 ÷ 32 = 0.1 3.2 ÷ 16 = 0.2 5 Divide by the smallest number 0.1 ÷ 0.1 = 1 0.2 ÷ 0.1 = 2 6 Write the formula SO2 2 more rows ...

 Main Topics
Main Topics